UCR Biosafety Manual
The UCR Biosafety manual defines the responsibilities, procedures, and guidelines for the safe handling, use, and disposal of biohazardous materials in research and teaching activities performed at UCR. The Biosafety Manual compliments the UCR Exposure Control Plan which contains additional policies and procedures for UCR personnel exposed to blood or other potentially infectious material.
If you have questions, please contact EH&S Biosafety at (951) 827-5528 or ehsbiosafety@ucr.edu
- Campus Biosafety Manual
- Document History
- 1. Purpose and Scope
- 2. Roles and Responsibilities
- 3. Biohazardous Research Approval Process
- 4. Laboratory Evaluations
- 5. Risk Groups
- 6. Biosafety Principles
- 7. Animal Biosafety
- 8. Plant Biosafety
- 9. Arthropod Biosafety
- 10. Personal Protective Equipment (PPE)
- 11. Laboratory Equipment
- 12. Sharps Safety
- 13. Transfer of Biological Materials
- 14. Decontamination, Disinfection, and Sterilization
- 15. Select Agents
- 16. Exposures and Injuries
- 17. Spill Procedures
- 18. Waste Management
- 19. Training
- Appendix A. Regulations, and Guidelines
- Appendix B. Links and Documents
- Acknowledgement Form
The UCR Biosafety Manual applies to all laboratories that use, store, or handle all biohazardous materials including recombinant/synthetic nucleic acids, human/animal/plant pathogens, and human and non-human primate materials. The Biosafety Officer will review the Biosafety Manual as least annually and update it as necessary.
| Date | Description of Changes |
Type of Change
(Review or Revision)
|
| 3/18/2020 | Revised and posted to EH&S website by Nick Noriea | Revision |
| 3/15/2021 | Reviewed by Tran Phan - no changes | Review |
| 3/15/2022 | Revised for change of Institutional Biosafety Committee administration from Office of Research Integrity to Environmental Health & Safety. Verified and updated references. Revised by Tran Phan | Revision |
| 3/10/2023 | Verified and updated figures and links. Revised by Rob Miers |
Revision |
| 3/26/2024 | Reviewed and updated link to Kuali system by Tran Phan | Revision |
| 4/30/2025 | Revised by Tran Phan to add IRE section under Roles and Responsibilities. Updates to sections VII (Occ Health), XIV (Sterilization), XVII (Spill Procedures), XVIII (Waste Management), and XIX (Training) | Revision |
I. Purpose and Scope
The purpose of the UC Riverside (UCR) Biosafety Program is to define and implement biological safety policies and practices to safeguard all UCR personnel, the community, and the surrounding environment from exposure to biohazardous materials. The policies and procedures are defined by the UCR Biosafety Program to comply with all federal, state, and UC requirements.
The UCR Biosafety Manual defines the responsibilities, procedures, and guidelines for the safe handling, use, and disposal of biohazardous materials in research and teaching activities performed at UCR. The Biosafety Manual compliments the UCR Exposure Control Plan (ECP) which contains additional policies and procedures for UCR personnel exposed to blood or other potentially infectious material.
All UCR Principal Investigators (PIs), researchers, and UCR personnel who may enter biohazardous research areas should adhere to these biological safety policies and procedures in the conduct of their research and in the management of their laboratories.
II. Roles and Responsibilities
INSTITUTIONAL BIOSAFETY COMMITTEE (IBC)
The National Institutes of Health (NIH) requires the establishment of an Institutional Biosafety Committee (IBC) at any institution that receives NIH funding. At UCR, the IBC is appointed by the Office of Research Integrity (ORI) under the auspices of the Vice Chancellor for Research and Economic Development (VC-RED). Administrative support for the IBC is provided by Environmental Health & Safety (EH&S).
The IBC is a faculty-led review committee responsible for approval and oversight of activities involving the storage and use of biohazardous materials. The IBC consists of at least five individuals so selected that they have experience and expertise in recombinant/synthetic nucleic acid (r/sNA) technology and can assess the safety of biohazardous experiments. The committee must have at least two community members who are not affiliated with UCR, an appropriate recombinant or synthetic DNA expert, a plant or animal containment expert (when applicable), and the institutional Biosafety Officer (BSO). The IBC membership is primarily composed of UCR faculty with expertise and capability to identify potential risks to public health or the environment.
The IBC is responsible for:
- Ensuring research conducted at UCR is compliant with the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (hereinafter as “NIH Guidelines”)
- Drafting and establishing campus biosafety policies and procedures for proper handling of biohazardous materials
- Reviewing individual research proposals for biosafety concerns
The IBC meets on the final Thursday of each month to review proposals from faculty. For more information, visit https://ehs.ucr.edu/ibc
ENVIRONMENTAL HEALTH AND SAFETY (EH&S)
Environmental Health and Safety oversees the Biosafety Program at UC Riverside.
EH&S is responsible for:
- Provide administrative support for the IBC.
- Monitors compliance with university, CDC, NIH, OSHA, USDA and state policies regarding the use of recombinant/synthetic nucleic acids, potentially infectious materials, pathogenic agents of livestock and agriculture, and exotic/invasive organisms
- Advises researchers and UCR personnel on appropriate safe work practices and procedures, containment controls, and personal protective equipment required for experimental protocols.
- Reviews and approves of transfer of biohazardous materials by PIs.
- Along with the IBC, reviews and approves all use of biohazardous materials.
- Provides training for proper handling, storing, disposing, and transporting of biohazardous materials in pursuant to regulatory requirements.
- Conducts periodic inspections of laboratories to ensure practices and procedures are rigorously followed and facilities are acceptable.
- Develops emergency procedures for occupational exposures and biohazardous spills.
- Investigates all reported accidents which may result in personnel or environmental exposure to biohazardous materials.
- Designate a Biosafety Officer (BSO) as defined by the NIH guidelines with duties including briefing the Institutional Biosafety Committee (IBC) on biohazardous research.
PRINCIPAL INVESTIGATOR (PI)
The Principal Investigator (PI) is directly and primarily responsible for the safe operation of their laboratory and for compliance by all their laboratory personnel with all UCR biosafety policies and procedures. The PI’s knowledge and judgement are critical in assessing risks and appropriately applying campus biosafety guidelines.
Principal Investigators (PIs) who wish to perform research using biological materials should submit a Biological Use Authorization (BUA) application to the IBC for approval prior to beginning work.
The PI is responsible for the following:
- They will not initiate research using biohazardous material and/or recombinant/synthetic nucleic acids until all applicable requirements outlined in this manual are met.
- They will consult and refer to NIH Guidelines and CDC’s Biosafety in Microbiological and Biomedical Laboratories (BMBL) to determine the appropriate Risk Group classification of the microorganisms to be used, and that they will use the prescribed microbiological practices and laboratory techniques required by the biosafety level assigned to their work by the IBC.
- They will report immediately to the BSO all deviations of the policies and procedures and all significant research-related accidents (spills, needle-sticks, exposure, injuries, etc.) which result in overt or potential exposure to infectious materials, or their release into the environment, whether contained within the laboratory or not.
- They are prepared to implement methods for dealing with accidents, spills, and personnel contamination.
- They maintain all appropriate state and federal permits required for certain animal and plant pathogens, exotic organisms, and plant pests prior to beginning work. PIs are strongly encouraged to contact EH&S Biosafety program at ehsbiosafety@ucr.edu for help in obtaining local, state, and federal permits.
- They will obtain the appropriate permits and Materials Transfer Agreements (MTA) for all appropriate importation, exportation and interstate shipping requirements for certain biological materials.
Prior to beginning research, the PI shall:
- Submit a BUA application for research and receive appropriate approval for all research projects.
- Submit BUA application amendment for any protocol changes that modify previously approved BUA procedures or alter the list of biohazards upon which approval was originally based.
- Determine the usefulness of serological screening, the requirements of medical surveillance, and the availability of vaccination for Risk Group 2 and 3 agents. Inform the laboratory staff of any precautionary medical practices advised or requested, such as vaccination or antibody titers. Where appropriate, the Hepatitis B vaccination should be offered free of charge to the employee.
- Assure that personnel working with infectious agents or biohazardous materials are appropriately trained, have a complete understanding of the hazards involved, and are proficient in the practices and techniques required for the safe handling of such materials.
During research, the PI shall:
- Appropriately supervise the performance of their staff to ensure that the required safety practices and techniques are employed.
- Investigate and report in writing to the IBC any significant biosafety problems pertaining to the pursuit of the research goals, specifically, new information which was not available at the time of the application.
- Correct any conditions or behaviors that might expose their personnel or release of biohazardous materials into the environment.
- Implement the procedures prescribed for dealing with laboratory incidents/accidents.
LABORATORY PERSONNEL
All personnel working in UCR research facilities, regardless of employment status, are responsible for the following:
- Follow all appropriate laboratory practices as outlined in this manual.
- Abide by all UC Riverside regulations and policies regarding safe conduct of research.
- Report all incidents, accidents, and possible unsafe work conditions to https://ehs.ucr.edu
- Familiarize and follow all protocols related to organisms and materials used in the laboratory regardless of whether or not they work directly with them.
- Follow all emergency procedures established by EH&S and the Principal Investigator (PI).
- Complete all appropriate training and verify documentation of training as required by EH&S.
III. Biohazardous Research Approval Process
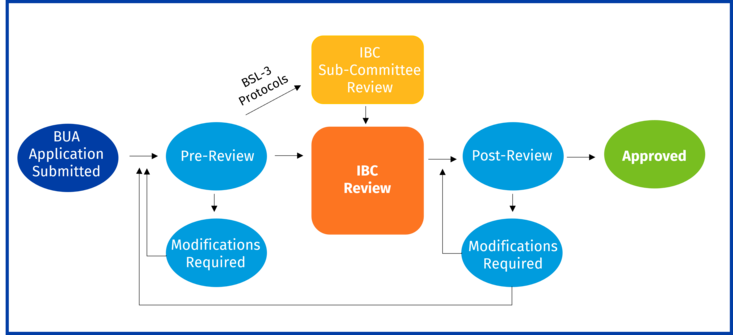
All biohazardous research at UCR requires an approved Biological Use Authorization (BUA) prior to beginning research. Principal Investigators who plan to carry out research involving the use of biohazardous materials need to complete and submit a BUA through the online portal prior to beginning work. The BUA need to be approved prior to initiation of research.
BUA applications should be submitted at least three weeks before the scheduled meeting and have adequately addressed any issues raised during the pre-review. The IBC reviews all research involving infectious agents or for which sections III-A,III-B, III-C, III-D, or III-E of the NIH Guidelines apply. Exempt work (section III-F of the NIH Guidelines without any other biohazards under the purview of the IBC) requires BUA submission and Biosafety pre-review only.
WHAT REQUIRES A BUA?
A BUA submission is required if working with any biohazardous material.
Biohazardous material covered under the IBC Charter includes:
- Recombinant or Synthetic Nucleic Acids (r/sNA)
- Human or Non-Human Primate Material (including blood, tissue, body fluids, and organs)
- Immortalized/Established cell lines from humans (e.g. Caco2, HEK293) or non-human primates
- Infectious agents (including Risk Group 1 agents that are infectious to vertebrates other than humans)
- Toxins of biological origin
- Select Agents and Toxins as defined by CDC and USDA/APHIS, including federally-exempt quantities
- Cellular penetrating peptides and other peptides capable of infecting living cells
- Transgenic animals in which the genome will be altered by stable introduction of recombinant DNA into the germ-line (e.g. making founder lines and breeding existing lines to create new genetic lines, but NOT including purchasing or maintaining already established lines)
- USDA-regulated plant pests
- Noxious weeds or plants that can outcross with noxious weeds
- Human Gene Transfer
- Wild caught animals
BUA REVIEW
New BUA submissions are processed by EH&S Biosafety program (EH&S) in Pre-Review. During Pre-Review, EH&S will review the BUA and may request the PI to modify the BUA. EH&S will make a formal determination of whether the BUA meets exempt status. For more information on the BUA approval process including Pre-Review, see https://ehs.ucr.edu/laboratory/biosafety/bua.
Once the BUA has completed Pre-Review, EH&S places it on the next IBC agenda to be formally reviewed by the Institutional Biosafety Committee (IBC). The IBC will vote whether to request additional information/modifications, decline, approve as is, or approve with conditions/stipulations. Only after the PI has been formally approved can research be initiated.
Exempt Research
All research involving r/sNA needs to be submitted in a BUA. Exempt research is research that only contains r/sNA under section III-F of the NIH Guidelines and contain no other biohazards. The PI does not determine exempt status, EH&S will notify the PI in pre-review if the BUA qualifies as exempt.
APPLYING FOR BUA
Preparing for a BUA
Prepare to establish the following when preparing a BUA for submission:
- Describe the scope of work to be conducted with biohazardous materials
- Propose the biosafety level of containment
- Propose the applicable sections of the NIH Guidelines
- Identify the researchers, lab workers and collaborators
- Identify the approved work locations
- Specify the safe work practices and procedures that should be adhered to
Submitting a BUA
The UCR EH&S website has guidance for PIs to submit new BUAs, renew expiring BUAs, or amend current BUAs: https://ehs.ucr.edu/laboratory/biosafety/bua
BUA DEFICIENCIES
Federal law requires significant deviations of NIH guidelines to be reported to NIH Office of Science Policy (OSP) within 30 days. Potential violations of NIH guidelines include research without prior IBC approval and failure to adhere to approved procedures and requirements.
Significant BUA deviations require the PI to stop work immediately. EH&S will issue the work-stoppage order to the PI. The PI may not continue work covered under the work-stoppage until formal notice to resume work.
Examples of BUA Deficiencies:
- Failure to obtain IBC approval prior to initiating work involving biohazardous materials
- Failure to amend BUA to include new biohazardous materials not described on the approved BUA
- Failure to follow procedures and policies established by the IBC and approved BUA
- Continuance of work after BUA expiration
- Continuance of work after work-stoppage notification by the IBC
- Significant research-related accidents resulting in overt exposure to personnel
If you believe you are possibly in violation of the NIH guidelines, please contact the Institutional Biosafety Committee (ibc@ucr.edu) or EH&S (ehsbiosafety@ucr.edu).
IV. Laboratory Evaluations
EH&S conducts both scheduled and unannounced evaluations of laboratories on a regular basis. Evaluations are not limited to biosafety, but rather encompass additional research-related categories such as chemical, fire and life, radiation, and general safety. A typical evaluation of a BSL1 or BSL2 laboratory will include, but may not be limited to, evaluation of the following items for compliance and/or accuracy:
Administration
- Emergency procedures and phone numbers posted
- Active personnel listed on BUA
Labeling
- Biohazard labels present at the entrance to the laboratory, on equipment, and waste containers
- Biohazard labels on secondary containers for red-bag biohazard waste
Administrative Controls
- Use of appropriate Personal Protective Equipment (PPE)
- Adherence to applicable medical surveillance program(s)
- Safety practices followed for aerosol-generating procedures
- Safe usage of sharps
- Decontamination of work areas
Equipment
- Biosafety cabinet annual certification and work practices
- Eyewash present and inspected
Personnel Training
- Biosafety Training
- Bloodborne Pathogens Training (if applicable)
- Hazardous Materials and Waste Management
- Laboratory Safety Fundamentals
- Fire Extinguisher Training
- Lab-specific Training
Waste Handling
- Use of appropriate biohazard bags used for waste
- Use of appropriate red rigid sharps bins for biohazardous sharps waste
- Proper disposal of dry and liquid biohazardous waste
- Decontamination procedures followed
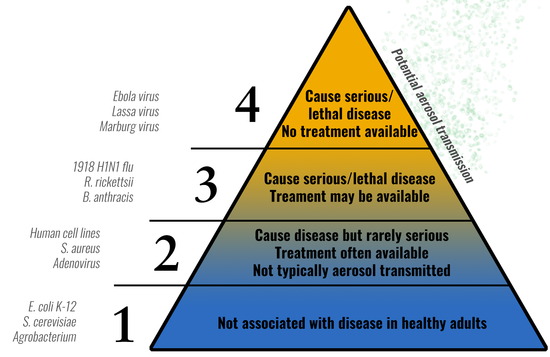
V. Risk Groups
When evaluating biohazards, it is helpful to categorize the risk present when handling various infectious agents. Categorizing infectious agents into Risk Groups (RG) allow researchers and biosafety professionals to anticipate expected procedures and practices necessary to work safely with that agent.
The principal hazardous characteristics of an infectious agent are:
- Capability to infect and cause disease in susceptible human/animal host
- Virulence as measured by severity of disease
- Availability of preventative measures (e.g. vaccines) and effective treatments
The NIH Guidelines established a classification of infectious agents (viruses, bacteria, fungi, parasites, etc.) into 4 risk groups (RG1 – RG4) using the characteristics listed above. The risk group increases with the risk of the agent
VI. Biosafety Principles
The Biosafety in Microbiological and Biomedical Laboratories (BMBL) manual describes four Biosafety Levels (BSL): combinations of laboratory safety practice and techniques, safety equipment and laboratory facilities. The Biosafety Levels were chosen to represent the conditions under which the agents can be ordinarily handled safely.
Biosafety levels are not equivalent to risk groups. Risk groups aid in determining which biosafety level to apply to the work being performed on a microorganism.
Biosafety Level 1
BSL-1 represents a basic level of containment that relies on standard microbiological practices only and a sink for basic hand washing. BSL-1 labs are appropriate for undergraduate/graduate teaching labs and for other labs in which work is done with defined microbes that are not known to cause disease in humans.
Biosafety Level 2
BSL-2 is the minimum biosafety level required to work with human pathogens. BSL-2 laboratories may be appropriate for a spectrum of moderate-risk agents associated with diseases of varying severity.
Biosafety Level 3
BSL-3 is required for work with indigenous or exotic agents with a potential for respiratory transmission and which may cause serious or potentially lethal infection. At this level emphasis is placed on aerosol transmission safeguards.
Biosafety Level 4
BSL-4 applies to work with dangerous and exotic agents that pose a high individual risk of life-threatening disease, may be transmitted via the aerosol route, and for which there is no treatment or intervention.
There are no Biosafety Level 4 labs on the UC Riverside campus or in the University of California system.
STANDARD MICROBIOLOGICAL PRACTICES (BSL-1)
All labs which contain biohazardous materials should adhere to standard microbiological practices. These practices and procedures encompass all the requirements of Biosafety Level 1 (BSL1).
- The laboratory supervisor can control access to the laboratory.
- The campus-wide biosafety manual should be available and accessible.
- Laboratory personnel should wash their hands after handling cultures, removing gloves and before leaving the laboratory.
- Eating, drinking, smoking, handling contacts lenses, application of cosmetics, and storing food or beverage for human consumption are prohibited.
- Pipetting by mouth is prohibited.
- Animals and plants not involved in the experiment are not permitted in the laboratory.
- Policies for handling sharps such as needles, blades, pipettes, and broken glass are implemented. Use of sharps is reduced as much as possible.
- Needles should not be bent, cut, removed from disposable syringes, or otherwise manipulated.
- Disposable sharps should be placed in specially designed puncture and leak-proof sharps containers and disposed of appropriately as medical waste.
- Non-disposable sharps should be placed in a hard-walled container for transport to decontamination, preferably by autoclaving.
- Broken glass should never be handled directly. Use tongs, forceps, or dustpan and brush. Use plastic instead of glass whenever possible.
- All laboratory procedures should be performed to minimize the creation of aerosols or splashes.
- Decontaminate all work surfaces after completing work, or after splashes or spills.
- Decontaminate all potentially biohazardous materials before disposal by an effective means.
- If decontamination of materials occurs outside the immediate laboratory, materials to be decontaminated should be packed in accordance with all applicable, local, state, and federal regulations and transported in durable, leak proof containers that are secured prior to transport.
- Signage incorporating the biohazard symbol should be posted when infectious agents are present.
- The laboratory supervisor should post safety procedures and equipment required for entry into animal housing areas containing known or potentially infected animals.
- An effective integrated pest management program is required.
- The laboratory supervisor should ensure that all laboratory personnel receive and annually refresh:
- Training appropriate to their duties
- Training on appropriate laboratory precautions
- Training on exposure evaluation procedures
- Training whenever a new hazardous material or procedure is introduced
Standard Laboratory Design
All UCR laboratories should adhere to the UC Lab Safety Design Manual. All UCR laboratories designated BSL1 or higher should fulfill the following criteria:
- Laboratories should have doors to isolate them from non-laboratory areas. Doors should be kept closed when experimental animals are present.
- Laboratories should be kept neat; good housekeeping procedures should be in place and in regular use.
- Laboratories should have a sink for hand washing. The sink should be located near the laboratory exit. It may be manually, hands-free or automatically operated.
- Laboratory furniture should be secured and appropriate for anticipated loads and uses. Spaces between benches, cabinets, and equipment should be accessible for cleaning and decontamination.
- Bench tops are impervious to water, moderate heat and chemicals.
- Laboratories are designed for ease of decontamination (e.g. no carpets, sealed surfaces, no unreachable areas, etc.)
- Seating in the laboratory should be covered with a non-porous material that can easily be cleaned and decontaminated.
- All laboratory windows to the outside should be fitted with fly screens.
- All laboratory spaces shall exhaust 100% of the air to the outside. Recirculated air is not allowed.
BIOSAFETY LEVEL 2 (BSL-2)
Biosafety level 2 builds upon BSL1 by adding additional special practices and safety equipment.
Special Practices
- All persons entering the laboratory should be advised of the potential hazards and should meet specific entry and exit requirements.
- Laboratory personnel are provided with medical surveillance and offered immunizations for agents handled or potentially present in the laboratory.
- The supervisor should ensure that laboratory personnel demonstrate proficiency in standard and special microbiological practices including disinfection and spill response before working with any biohazards.
- Potentially infectious and/or recombinant materials should be in durable leak proof containers during collection, handling, processing, storage or transport within a facility.
- Laboratory equipment should be routinely decontaminated as well as after any potential contamination.
- Equipment should be decontaminated before repair, maintenance, or removal from the laboratory. Guidance documents can be found at https://ehs.ucr.edu/laboratory
- Potential infectious agent exposure incidents are immediately evaluated and treated per the laboratory biosafety manual. They are reported to the supervisor. Medical evaluation, treatment and surveillance are provided as appropriate.
- All laboratory procedures that may generate aerosols should be performed in a properly certified biological safety cabinet or other physical containment device.
- Centrifugation of potentially infectious material requires the use of safety cups if the centrifuge is outside of a biosafety cabinet.
- Sonication of potentially infectious material requires the use of a biosafety cabinet or sealed cup horn.
Safety Equipment
- Primary barriers such as biosafety cabinets should be used when handling biohazardous materials. This may include pipetting, grinding, centrifuging, shaking, blending, sonicating, opening containers of infectious material, intranasal animal inoculation, harvesting infected tissues from animals or eggs, or if high concentrations are used.
- Biosafety cabinets should be certified at least annually, and whenever moved or after repair work is done, if used to contain biohazards.
- Biosafety cabinets are installed so that laboratory air movement does not disrupt cabinet operation; away from doors, windows, high-traffic areas, ventilation registers and other disruptions.
- HEPA-filtered cabinet exhaust may be re-circulated to the laboratory if the cabinet is certified at least annually. The exhaust may also be ducted to the outside via either a thimble- or hard-connected exhaust duct.
- PPE should be disposed of with other contaminated waste or decontaminated before reuse. Contact lens wearers should also use eye protection.
- Double gloving may be appropriate. Recommend using darker color inner glove and lighter color outer glove to more easily identify breaks or tears in an outer glove.
Lab Design
- Doors should be self-closing and have locks
- Vacuum lines should be protected by HEPA filters that are cleaned or replaced as needed. Filter traps may be required.
- Autoclave, chemical disinfection, incineration or other approved decontamination method should be available in the facility for waste treatment prior to disposal.
BIOSAFETY LEVEL 3 (BSL-3)
Biosafety Level 3 (BSL3) builds upon BSL2 with several additional requirements. Laboratories must have anterooms, negative airflow, and single-pass filtered exhaust. Work is restricted to biosafety cabinets. The facility design includes several additional standards and rigorous safety and security checks are required for personnel working at BSL3.
For additional information on High Containment (BSL3) Laboratories, see Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th edition or contact ehsbiosafety@ucr.edu.
VII. Animal Biosafety
All research involving animals at UCR must be reviewed and approved by the UCR Institutional Animal Care and Use Committee (IACUC) using the Animal Use Protocol (AUP). Animal research containing biohazards need to also be described in an approved Biological Use Authorization (BUA). When working with biohazards and animals, consider the following as part of the research risk assessment:
- Dosage and route of administration of biohazards to be used in animal model
- Any infectious agents associated with the animal model (e.g. Herpes B virus, C. burnetii)
- Shedding of infectious organisms/biohazards from the animal through blood, feces, urine, etc.
- Potential allergies
For more information about the IACUC, contact iacuc@ucr.edu or visit https://research.ucr.edu/ori/iacuc
ANIMAL CONTAINMENT PRINCIPLES
The Animal Biosafety Level (ABSL) required for given animal research is assigned by the Institutional Biosafety Committee (IBC). Like general laboratory biosafety levels, animal biosafety levels are assigned 1 to 4 and reflect a standard set of practices, equipment, and containment for the safe handling of biohazardous materials in animal research.
Animal Biosafety Level 1 (ABSL-1)
Suitable for work in animals involving well-characterized agents that are not known to cause disease in immunocompetent adult humans and present minimal potential hazard to personnel and the environment.
Animal Biosafety Level 2 (ABSL-2)
Suitable for work involving laboratory animals infected with agents associated with human disease and pose moderate hazards to personnel and the environment.
Animal Biosafety Level 3 (ABSL-3)
Suitable for work with laboratory animals infected with indigenous or exotic agents that present a potential for aerosol transmission and agents causing serious or potentially lethal disease.
LABORATORY OCCUPATIONAL HEALTH PROGRAM
All UCR animal users are offered enrollment in the UCR Animal Occupational Health and Safety Program. In part, enrollment requires annual evaluation by a medical occupational health specialist. Contact EH&S Occupational Health for further assistance regarding the Occupational health program.
VIII. Plant Biosafety
The research of plants and their associated organisms present interesting and unique containment concerns. While the risk of human infection is lower, environmental risk can increase. The unintentional release of a transgenic arthropod or exotic weed can have significant effects on the surrounding environment.
Plant-related biohazards include:
- Transgenic plants
- Plants associated with transgenic microbes or arthropods
- Noxious weeds or plants that can outcross with noxious weeds
- USDA recognized plant pests
- Plants containing the genome of any non-exotic infectious agent
- Plants containing exotic agents
- Plants encoding the sequence of potent vertebrate toxins (LD50 < 100 ng/kg)
- Plants associated with non-exotic microorganisms that have a recognized potential for serious detrimental impact on managed or natural ecosystems
Research with plant-associated biohazards may also require permits with conditions specified by USDA/APHIS or CDFA. The EH&S biosafety program will assist PIs in application and compliance for plant-related regulatory permits. Approved USDA/APHIS or CDFA permits are not a substitute for BUA approval.
For experiments in which plants are grown in BSL-1 and BSL-2 laboratory settings, containment practices should be in correspondence with standard biosafety levels. This includes plant tissue culture rooms and growth chambers within laboratory facilities. Some additional containment procedures may be required by the IBC prior to research initiation.
GREENHOUSE BIOSAFETY
For research that requires research plants of a size, number, or growth requirements that preclude the use of laboratory containment conditions, additional precautions need to be taken. UCR employs over a thousand acres of field research with greenhouses, screen-houses, and two field stations. The UCR main campus additionally contains several greenhouses on site. Recommended containment practices in these greenhouses is described by the Plant Biosafety Levels (BSL-P).
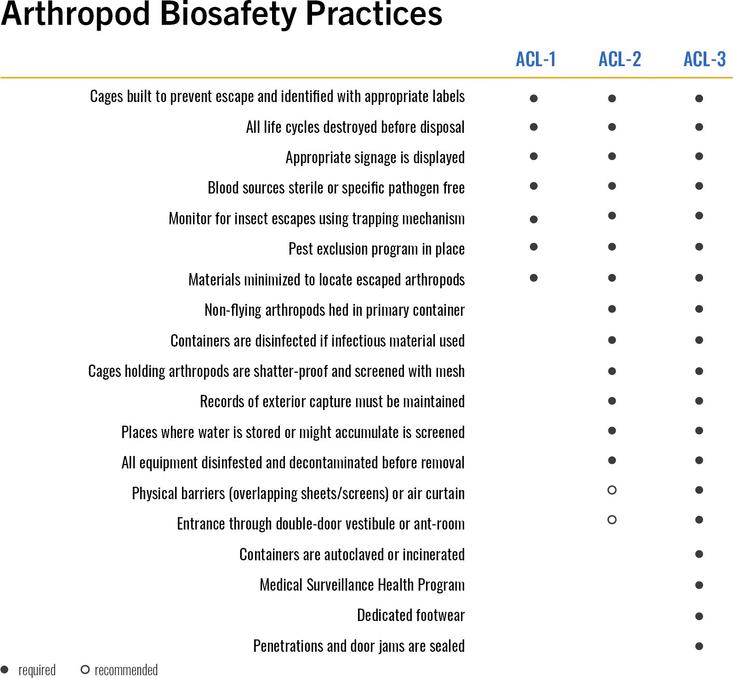
IX. Arthropod Biosafety
Laboratory research involving arthropods present challenges distinct from typical microbiological and biomedical laboratories. Escape and environmental protection are significant concerns. Many arthropods may not be infectious but act as a vector for diseases in the environment. Some arthropods are deleterious to livestock or agriculture (e.g. a plant pest). The small size and motile characteristics of many transgenic arthropods require additional administrative, equipment, and design safeguards to prevent release.
What Arthropod research requires a BUA?
Arthropod-related biohazards include:
- Transgenic arthropods
- Exotic arthropods
- Arthropods associated with exotic agents
- Arthropods that transmit pathogens of public health importance
- Arthropods that are known plant pests as defined by the USDA
- Arthropods associated with transgenic microbes, plants, or other associated animals
Arthropods to be considered for risk assessment include
- Insects (Diptera, Hemiptera, Phtiraptera, Siphonaptera)
- Arachnids (Acari)
All life cycle stages are considered under the term arthropod: eggs, larvae, nymphs, and adults.
ARTHROPOD RISK ASSESSMENT
When containment is required for arthropod research, the following should be considered:
- Is the arthropod exotic, and if so, is it likely to establish locally if it escapes?
- Does the arthropod have acquired or characterized insecticide-resistance (through genotype or transgenics)?
- Is the arthropod a vector for any infectious agents found in the environment?
- Are disabled strains available, whose viability after escape would be limited?
- Is the arthropod transgenic? If so, does this alter capability of pathogen transmission or environmental persistence?
X. Personal Protective Equipment (PPE)
The UC Personal Protective Equipment (PPE) policy is designed to prevent workplace injuries and illnesses for academic appointees, staff, students, and visitors. General laboratory PPE is determined by the PI’s Laboratory Hazard Assessment. Risks involving biohazards may require additional PPE beyond what the Laboratory Hazard Assessment describes. All approved Biological Use Authorization (BUA) describe additional PPE requirements for working with the associated biohazards.
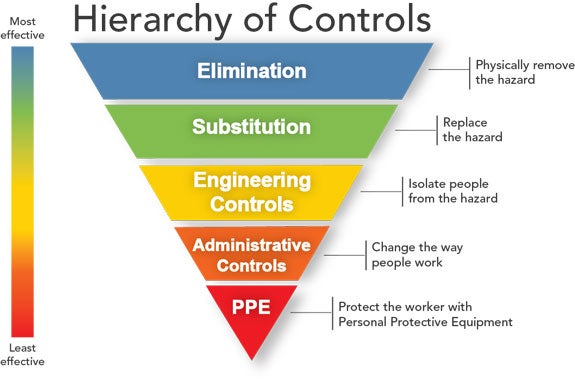
Before discussing PPE, one should become familiar with the NIOSH Hierarchy of Controls that are used to protect workers. The top controls are more effective and preventative than controls at the bottom.
PPE is considered the last line of defense and the least effective for protecting the worker. PPE should never substitute for safe procedures, appropriate safety equipment, or safer alternatives.
GENERAL ATTIRE
All researchers need to wear full length pants (or equivalent) and closed toe/heel shoe attire at all times while entering or occupying laboratory areas. The area of skin between the pants and shoe should not be exposed.
Lab Coats
All researchers working in labs with biological hazards need to wear a lab coat. Lab coats are supplied free of charge by UCR Environmental Health and Safety through the Laboratory Hazard Assessment Tool. There are many types of lab coats available; researchers working with biohazards are recommended the traditional white lab coat or the barrier/fluid-resistant white lab coat. Some biohazards may require additional protection such as disposable gowns.
Lab coats should not be taken home. Researchers working with biohazards may contaminate their lab coats during normal usage. Due to the high risk of lab coats acting as fomites, lab coats should be kept at work. EH&S supplies two lab coats to each researcher to ensure that the researcher may continue to wear the appropriate lab coat while the other is laundered.
Laundering Lab Coats
Lightly soiled laboratory coats should be cleaned and properly laundered. Laboratory coats which are contaminated with infectious materials should be appropriately decontaminated by soaking the contaminated material in freshly-made 10% bleach for 30 minutes or disposed of as biohazardous waste.
Gloves
Gloves should be worn at all times when in contact, or could potentially come in contact, with any biohazardous material in the laboratory. Standard latex or nitrile gloves are sufficient for working with biological hazards, however if chemical hazards are involved these gloves may not be sufficient. Many types of gloves offer biological protection but may not offer equal levels of chemical protection.
When working with chemical and biological hazards, always consider the chemical protection first.
Autoclave gloves should always be used for removing hot material from autoclaves. Cryo gloves should be used for handling liquid nitrogen.
Eyewear
Researchers working with biological hazards may consider several types of eye protection including safety glasses, safety goggles, and face shields. Researchers are required, at a minimum, to wear safety glasses while working with biohazards.
Surgical masks
Surgical masks are not respirators. Surgical masks are designed to protect the user’s nose and mouth from liquid droplets, they are not used to protect against aerosols. Surgical masks are used to prevent dust/particle inhalation.
RESPIRATORY PROTECTION
Respirators are devices designed to protect the wearer from airborne hazards such as airborne transmissible microorganisms. The selection of respirator depends on the type of hazard potentially present and potential for aerosol production.
N95
N95 respirators are certified to block 95% of particles 0.3 µm or larger (0.3 µm is roughly the size of a single virus). All researchers required to wear N95 need to be fit tested and complete an occupational medical health questionnaire to ensure that the N95 poses no additional safety hazards to the user. To request a medical questionnaire or schedule a fit test, contact EH&S at ehsih@ucr.edu. Some researchers may not be required to wear a respirator but choose to wear a N95. For volunteer use forms for N95, contact ehsih@ucr.edu.
Powered Air Purifying Respirator (PAPR)
The PAPR provides one of the highest levels of protection against biohazards. PAPRs are commonly found in high containment laboratories to protect against aerosolizable infectious material. A PAPR contains a battery-powered blower, facemask, breathing tube, and HEPA filter. The air is blown across the filter and deliver breathable air into the facemask. The filter cartridge may be exchanged to filter chemical vapors and toxic gases. EH&S requires annual training for all PAPR users.
XI. Laboratory Equipment
Laboratory equipment represent the engineering controls used to control researcher exposure to biohazards. Many pieces of equipment function as primary containment when used with infectious material. Used correctly, laboratory equipment can provide high levels of protection for both the user, their research, and the environment.
TYPES OF PROTECTION
There are 3 types of protection that equipment can provide:
- Personnel Protection: To protect people from hazards
- Product Protection: To protect experimental materials from contamination
- Environmental Protection: To protect the surrounding environment from hazards
When considering equipment for the lab, researchers should always examine what kind of containment they require versus what kind of containment the equipment provides.
BIOSAFETY CABINETS
Biological Safety Cabinets (BSCs) are designed to provide personnel, environmental and product protection when appropriate practices and procedures are followed. There are three classes of BSCs. At UC Riverside, almost all biological safety cabinets approved for use of biohazards and human material are Class II. Please contact the biosafety office at ehsbiosafety@ucr.edu if you plan to use a Class I or Class III cabinet in your research. Class II cabinets can be further subdivided into room-exhausted (Class II-A) or ducted exhaust (Class II-B). Class II-A cabinets recirculate 70% of air back into the work zone while Class II-B cabinets recirculate either 0% (Class II-B2) or 30% (Class II-B1) of air into the work zone. Class II biosafety cabinets provide all 3 types of protection.
Figure XI-1. Types of Protection offered
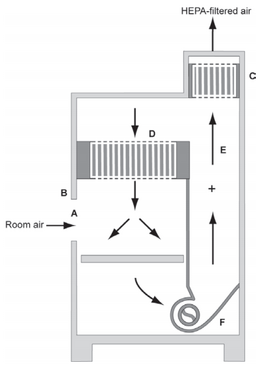
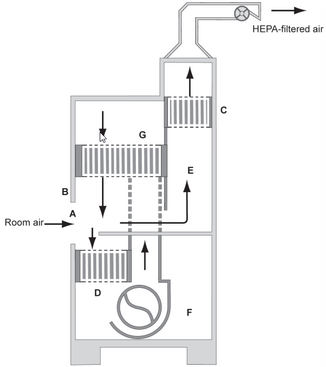
Figure XI-2. Classes of Type II Biosafety Cabinets
Figure XI-2. Class II-A1 Biosafety Cabinet. (A) front opening; (B) Sash; (C) exhaust HEPA filter; (D) return HEPA filter; (E) common plenum; (F) blower. (BMBL 5th ed)
Figure XI-3. Class II-B1 Biosafety Cabinet. (A) front opening; (B) Sash; (C) exhaust HEPA filter; (D) supply HEPA filter; (E) negative pressure dedicated plenum; (F) blower; (G) additional supply HEPA filter. (BMBL 5th ed)
PROCEDURE FOR USING A BIOSAFETY CABINET
Tips
- Never put your head inside the cabinet.
- 10% household bleach may be used safely in biosafety cabinets; however bleach should be followed by sterile water or 70% ethanol to prevent pitting to the stainless steel surface.
- The biosafety cabinet is only effective at the sash height it was certified at. If the sash is at an inappropriate level for your height, adjust the seat- never adjust the sash.
- Biosafety cabinets need to be certified annually to ensure correct operation. Any BSC used for biohazardous materials should be current on certification.
Before Use
- Wear appropriate PPE.
- Check certification sticker located on the exterior surface of BSC to confirm that BSC has been certified within the past 12 months. Notify PI if BSC certification exceeds 12 months. In this case, do not use BSC.
- Turn on cabinet blowers.
- Check the magnehelic gauge to ensure BSC is working properly.
- Raise the sash to the certified operating height. Wait at least 2– 5 minutes prior to beginning work to allow the BSC to purge.
- Do not ignore alarms.
- Adjust seat height so that researcher’s face is above the front opening.
- Using a squeeze bottle, spray the appropriate disinfectant onto interior surfaces of the BSC (work surface, side walls, back walls, front grill, inner glass of sash etc.). Do not put your head inside the BSC during cleaning.
- Wipe down the work surface area by starting at the left front corner at the grill and wiping across the front grill. Continue wiping horizontally across towards the back of the BSC (see Figure 11-4 – yellow arrows).
- Wipe down inner glass of sash by starting on the lower left corner and wiping up and down while moving horizontally (see Figure 11-4 – blue arrows).
Figure 11-4. Cleaning work surface (yellow arrows) and sash (blue arrows)
- Wipe down side walls and back of BSC (see Figure 11-5).
Figure 11-5. Cleaning side walls and back
- Dispose soiled paper towels into biohazard bag.
- Replace gloves. BSC is ready to be set up before commencing work.
- Place all materials towards the back of the work area away from front and rear grills. Leave at least 6 inches between work area and the side, front, and back grills.
- Set up BSC work flow from clean to dirty as shown in figure XI-6.
UV Lamps in Biosafety Cabinets
The CDC, NIH, NSF, and American Biological Safety Association (ABSA) agree that UV lamps are neither recommended nor required in a Biological Safety Cabinet.
All material inside a biosafety cabinet should be decontaminated with an approved disinfectant prior to removal, regardless if the material was treated with UV light.
VACUUM LINES
All vacuum lines used for biohazardous materials should be protected with a vacuum line HEPA filter placed closest to the vacuum line port. Attach an aspiration flask (“trap”). A second inline aspiration flask is recommended. The aspiration flask(s) should be placed into a non-absorbent, rigid secondary container. Styrofoam and cardboard are not appropriate for use as a secondary container.
Aspiration flasks need to be filled with full-strength household bleach with an amount 1/10th the volume of the flask. This ensures that even if the trap reaches capacity the minimum 10% bleach final concentration can be reached. Other disinfectants may be used if documented in the laboratory’s approved BUA.
CENTRIFUGE SAFETY
Centrifuges can cause serious hazard concerns due to the high speed at which centrifuges operate. Unbalanced centrifuge rotors can result in injury, even death. Sample container breakage can generate aerosols that may be harmful if inhaled.
To Avoid Mechanical Failure:
- Follow manufacturer instructions to avoid metal fatigue, distortion, or corrosion.
- Review the operating manual before using a centrifuge.
- Maintain and operate the centrifuge according to manufacturer instructions.
- Examine the centrifuge often for damage or poor maintenance.
- Properly train users. Post operating instructions that include safety precautions on the unit.
- Contact vendor in case of any problems.
To Avoid Rotor Failure:
High-speed rotor heads are at risk to metal fatigue. Failure to discard rotors after a predetermined amount of use can result in dangerous and expensive rotor disintegration. It is extremely important to avoid rotor failure. Follow these guidelines to reduce the risk of rotor failure:
- Ensure rotor is appropriate for the centrifuge.
- Always use matched sets of tubes, buckets, and other equipment.
- Use sealed rotors, sealed buckets, or a guard bowl with a gasket and cover, as well as safety centrifuge tubes (tube or bottle carrier with sealable cap or “O-ring” gasket cap).
- Follow manufacturer specifications for the rotors and tubes. Keep manual near the unit for easy reference.
- Follow the manufacturer’s maximum speed and sample density ratings designations to prevent stress failures.
- Make sure the rotor has come to a complete stop before opening the lid.
- Keep track of each rotor, recording the purchase date of each rotor, manufacturing date, serial number, the length of time and speed for each use.
- Discard rotors according to the manufacturer’s recommended schedule.
Minimizing Aerosols:
Aerosols are fine particles of liquid droplets produced when energy is applied to a liquid and such liquid escapes into the environment. Aerosols are potentially created during the process of centrifugation, incubation with shakers, filling centrifuge tubes, removing plugs or caps from tubes after centrifugation, decanting supernatant, or resuspending sedimented pellets. The highest concern for aerosol hazards is a primary container (such as an Eppendorf tube or 5mL conical) breaking during centrifugation.
Follow these guidelines to minimize aerosols when centrifuging biohazardous materials:
- Use safety centrifuge cups to contain potential spills and prevent aerosols.
- Fill and open centrifuge tubes, rotors, and accessories in a biosafety cabinet (BSC).
- Use sealed tubes and safety tubes with O-rings. Inspect O-rings and buckets before each use.
- Do not use aluminum foil or non-sealable materials to cap centrifuge tubes.
- Avoid overfilling centrifuge tubes to prevent caps from becoming wet.
- After tubes are filled and sealed, wipe the exterior of the tube with disinfectant.
- Properly balance buckets, tubes, and rotors before centrifugation.
- Do not decant or pour off supernatant. Use a mechanical device such as a micro pipette or an aspiration flask system with appropriate in-line HEPA filter to withdraw liquid.
XII. Sharps Safety
Many practices in research laboratories have the potential for sharps injuries. Biologically contaminated sharps are a significant source of biological exposures in the laboratory. Common sharps in the lab include needles, scalpels, razor blades, broken glass, lancets, glass slides, and fragile glass items (e.g. ampoules, Pasteur pipettes, and glass vials). Refer to UCR’s Exposure Control Plan for additional information on sharps exposures processes.
BEST PRACTICES
- Never shear or break needles or other sharps.
- Do not reuse disposable sharps.
- Always use a mechanical means to pick up broken glassware (tongs, forceps, or dustpan/brush)
- Never open, empty, or clean a sharps container in a manner that would expose someone to the risk of sharps injury.
Contaminated sharps should be immediately placed in sharps containers. EH&S provides chemical and biological sharps containers. Contact ehslaboratory@ucr.edu to request additional sharps containers.
NEEDLES AND NEEDLE-DEVICES
Any type of needle, including but not limited to hollow-bore needles are a significant cause of sharps injuries in the lab. Engineering sharps protection is a physical attribute built into the needle device which effectively reduces the risk of an exposure by a mechanism such as barrier creation, blunting, encapsulation, withdrawal, or other mechanisms.
Recapping or Bending Needles
Bending or recapping needles should not occur unless the procedure is performed using a mechanical device or one-handed “scoop” technique and it can be demonstrated that there is no feasible alternative or that the specific procedure requires such an action. For each device, the laboratory must describe the reason for the bending or recapping of sharps in an approved laboratory SOP or BUA.
Do not recap needles before disposal.
XIII. Transfer of Biological Materials
MATERIAL TRANSFER AGREEMENTS (MTA)
A Materials Transfer Agreement (MTA) is a legally binding contract between two organizations (e.g. universities, non-profit, industry) regarding the transfer of research materials. An MTA is used to define the rights of both the supplier and recipient. The UCR Office of Technology Commercialization (OTC) facilitates MTAs for campus research and intellectual property.
EH&S encourages all investigators receiving biological material from external sources to contact OTC at mta@ucr.edu to inquire if an MTA is recommended. Investigators can create an MTA using Kuali.
IMPORT / EXPORT OF BIOLOGICAL MATERIALS
Federal, state, and international law strictly regulate shipping, transport, and import of biological materials. Investigators must obtain all necessary permits prior to the importation of any biological material. It is strongly recommended that PIs contact EH&S Biosafety to obtain the proper permit and prepare for permit-related inspections.
Biological Material subject to import/export requirements
- Cell or tissue culture (both primary and immortalized)
- Cell or tissue culture products
- Microorganisms, including exotic and recombinant organisms
- Infectious material for humans, animals, plants, or arthropods
- Toxins or material encoding human toxins
- Tissue/Organs and their extracts
- Blood and plasma
- Transgenic organisms
- Plant pests
- Noxious weeds or plants that can outcross with noxious weeds
- Exotic plants and their associated microorganisms
- Exotic arthropods and associated microorganisms
Obtaining Permits
EH&S Biosafety can assist with obtaining state and federal permits required for importation/exportation of biological materials. EH&S can assist with permits including:
• PPQ 526 (move live plant pests, biological control agents, or noxious weeds)
• PPQ 586 (transfer plants or plant products through US)
• PPQ 587 (import plant or plant products)
• PPQ 588 (import restricted or not-authorized plant material)
• CDFA 66-026 (move and use live insects or plant pests or noxious weeds)
• VS16-6 (transport organisms or vectors or animal products)
• BRS 2000 (permit for movement or release of genetically engineered organisms)
• CDC Permit to Import Vectors or Agents of Human Disease
• CDC Permit to Import or Transport Live Bats
Let the EH&S Biosafety office help you in selecting and acquiring the required permits. Contact ehsbiosafety@ucr.edu for more information.
INTRACAMPUS TRANSFER OF BIOLOGICAL MATERIALS
Biohazardous agents should be properly handled, contained and labeled to transport between locations to prevent accidental release or exposure to unsuspecting persons outside of the laboratory. Refer to the Biological Materials Packing and Transport SOP.
Biohazardous agents should be placed in securely closed and labeled primary containers. The exterior of the primary container should be decontaminated prior to transportation.
The primary container should be placed in a covered, leak proof, shatterproof secondary container. The secondary container should be labeled with the biohazard symbol, the biohazardous agents present and the lab of origin. If it is transported by vehicle, the name and telephone number of the PI or other responsible person(s) should be included on the outside of the secondary container.
PACKAGING, SHIPPING, AND TRANSPORTATION
Federal (Department of Transportation, 49 CFR §171-175) and international agencies (IATA – International Air Transport Association) have in place regulations for shipping of dangerous goods by surface or air.
Any person involved in packaging, handling, shipping or transporting hazardous materials must receive training in the general requirements of handling hazardous materials. Initial training is required before performing any tasks associated with shipping hazardous materials and refreshed every 2 years.
Shipment Off-Campus (Domestic Shipment):
Three federal regulatory agencies specify requirements for packaging and shipping of biological materials. The United Nations publishes recommendations for packing and shipping biological materials, and both the International Civil Aeronautics Organization and International Air Transport Association (IATA) publish regulations based on the UN’s recommendations. The requirements for all these regulations are similar; therefore, most carriers elect to follow the IATA regulations set forth in their Dangerous Goods Regulations (DGR).
Depending on the material being shipped, there are various packing instructions that need to be adhered to. The following is an example of packing instructions for infectious substances:
Figure XIII. U.S. Department of Transportation - Example of Proper Packaging
Primary Container
- The specimen will be placed in a securely closed, watertight primary container. Stoppers and screw-capped tubes will be secured with waterproof tape.
- The exterior of the primary container will be decontaminated prior to transportation.
- The exterior of the primary container will be properly labelled with information regarding the contents of the container.
Secondary Container
- One (or more) primary container(s) may be placed within the secondary container as long as the total volume of the specimen does not exceed 50 ml. More than one primary container can be placed inside the secondary container as long as each primary container is wrapped/cushioned to prevent contact between primary containers.
- The absorbent material used within the secondary container should be sufficient to absorb the contents of the primary container(s), if it should leak.
- The secondary container should be free of contamination and labeled with the biohazard symbol.
Outer Container
- This container will be made of corrugated fiberboard, cardboard, or other material of equivalent strength.
- The interior of the outer container may be filled with coolant material such as ice packs or dry ice. DO NOT USE WET ICE FOR SHIPPING. The dry ice should be placed outside of the secondary container in the outer container with proper venting. An additional placard is required with the mass of dry ice indicated on the placard.
The exterior will be labeled with the special sticker as shown. Additional labeling may be required, including UN Category, volume, dry ice warning, and IATA packing instruction number.
- Prior to transport, the outer container should be sealed or secured in a manner as to make it leak-proof should the container be placed on its side.
- The package will be decontaminated before shipment.
International Shipments:
All domestic and international shipments of infectious substances require the use of packaging that has been tested and certified to carry such material. The certified packaging will have UN performance markings on the outside indicating that it has met performance tests. The shipper should include the name and telephone number of the person responsible for the shipment. Diagnostic specimens being shipped for the purpose of initial diagnosis are excluded from the regulations. However, diagnostic specimens known, or thought likely, to contain infectious substances are included.
Receipt of Packages:
Upon receipt of any packaged specimens, immediately check for leakage or damage.
If leaking:
- Isolate the package in a biosafety cabinet
- Place package in leak-proof, sealed container
- Submerge contents in 10% bleach or approved disinfectant for at least 30 minutes
- Dispose of as biohazardous waste.
- Contact EH&S if material was potentially infectious
XIV. Decontamination, Disinfection, and Sterilization
UCR laboratories with biohazards need to be sufficiently decontaminated in order to eliminate the possibility of transmission of infectious materials to researchers, the public, and the environment.
Decontamination renders an item safe to handle by reducing the level of contamination enough to be reasonably assumed free of transmission risk. Biohazard decontamination occurs through disinfection or sterilization.
Cleaning
Cleaning is the removal of foreign material from objects, normally accomplished using water with detergents or enzymatic products. Cleaning can be done manually or automatically. Cleaners such as ultrasonic cleaners do not remove microorganisms but can increase the efficacy of disinfectants. By itself, cleaning is not considered a valid method of decontamination for biohazardous materials.
DISINFECTION
Disinfection is the process that eliminates many or all pathogenic microorganisms, except bacterial spores, on inanimate objects. It is considered a less lethal process than sterilization (disinfectants do not kill spores or contain the “overkill” safety margin that sterilization provides)
Disinfection is used for surface decontamination and, at sufficient concentration, to decontaminate liquid biohazardous waste. Biohazardous liquid waste disinfected with bleach may be poured down the sewer as final disposal.
Choosing the right disinfectant
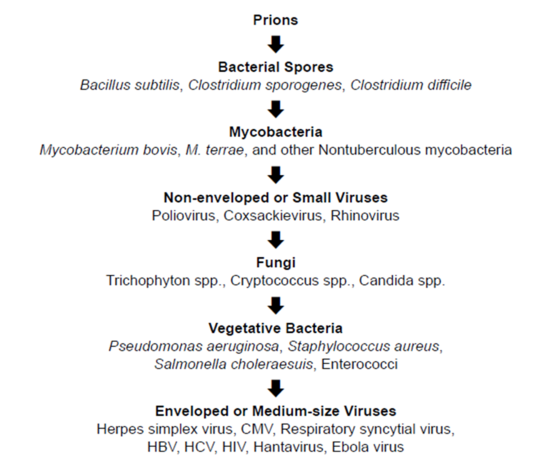
Microorganisms vary significantly in their resistance to disinfectants. Even when the agent is known, disinfectant effectiveness is controlled by a number of other factors:
• The type of microorganisms (see Figure XIV-1)
• The number of microorganisms
• Amount of organic and inorganic matter present (e.g. soil, blood)
• Presence of biofilms or other extracellular matrixes
• Temperature
• Contact Time
Figure XIV-1. Descending Order of Resistance to Germicidal Chemicals (BMBL, 6th ed.)
Disinfectant Classifications
The FDA and EPA classify disinfectants based on activity level:
High-Level Disinfectants. High level disinfectants are regulated by the FDA and can function as sterilants. High level disinfectants kill vegetative microorganisms, inactivate viruses, and may be sporicidal with prolonged contact times. They are classified by the FDA as “sterilant/ disinfectants”. FDA approved sterilant/disinfectants include peracetic acid, glutaraldehyde, formaldehyde, ethylene oxide, hydrogen peroxide, and ortho-phthalaldehyde.
Intermediate-Level Disinfectants. Intermediate level disinfectants are regulated by the EPA and are disinfectants that kill vegetative microorganisms, including Mycobacterium tuberculosis, all fungi, and inactivate most viruses. The EPA labels these products as “tuberculocidal”.
Intermediate level disinfects are the most common type used in the laboratory with biohazards. Intermediate level disinfectants include chlorine and chlorine compounds, alcohol, iodophors, and phenol.
Low-Level Disinfectants. Low level disinfectants kill most vegetative bacteria except M. tuberculosis, some fungi, and inactivate some viruses. They are separated from the intermediate-level by the lack of tuberculocidal activity. The EPA approves low-level disinfectants as “sanitizers” and “hospital disinfectant”. Low level disinfectants include Quaternary ammonium compounds and some iodine solutions.
STERILIZATION
Sterilization is the process that destroys or eliminates all forms of microbial life. Any item, device, or solution is considered to be sterile when it is completely void of all living microbes and viruses. Sterilization is not a specific mechanism but any procedure that reduces the probability of the microorganism from surviving on the treated item to be less than 1 in 106. This is the sterility assurance level. Sterilization is typically accomplished using heat or chemical vapors/gases.
Heat Sterilization
Heat sterilization is achieved by using an autoclave by applying wet heat (i.e. high-pressure steam) at temperatures above the normal boiling point of water and pressures above normal atmospheric pressure. Autoclaves are used to sterilize laboratory equipment or materials such as glassware, media, reagents, or waste.
Autoclaves at UCR are not registered with the county for medical waste autoclave certification. All medical waste and biohazardous materials should still be placed in the EH&S provided waste bins after autoclaving for final pick up and disposal by our third party vendor. Permit holders who are required to autoclave their waste prior to disposal should still place their autoclaved waste in the EH&S provided waste bins after autoclaving. Permit holders can request biological indicators for the Monthly Autoclave Testing through EH&S. If required by the permit, the PI is the responsible party to conduct the monthly testing of the autoclaves using Bacillus strearothermophillus biological indicators. An autoclave use log should be completed by each user for each autoclave cycle. Records should be kept for a minimum of three years.
Vapors and Gases
Vapors and gases are used in a closed system and under controlled conditions of temperature and humidity. Agents in this category include the aerosol, vapor, or gas phase of chlorine dioxide, glutaraldehyde, paraformaldehyde, ethylene oxide, peracetic acid, and hydrogen peroxide.
Vapors and gases are primarily used to decontaminate biosafety cabinets, animal rooms, and their associated systems, bulky or stationary equipment not suited to liquid disinfectants, instruments or optics that might be damaged by other decontamination methods, and rooms, buildings, and associated air-handling systems.
EH&S does not provide sterilization services. The primary UC service-provider for sterilization is Technical Safety Services (TSS). Due to the hazardous nature, contact EH&S Biosafety (ehsbiosafety@ucr.edu or x2-5528) prior to administration.
XV. Select Agents
Select Agents are materials that have been identified by the U.S. Government as agents that have a potential use in biological terrorism or warfare. The Federal Select Agent Program (FSAP) oversees the possession, transfer and use of select biological agents and toxins that have the potential to pose a severe threat to public, animal or plant health, or to animal or plant products in the United States and its territories. The FSAP requires government agencies, universities, research institutions, and commercial entities that possess, transfer, and use biological agents and toxins to register with the program and UCR IBC. Additional information can be accessed at http://www.selectagents.gov/index.html.
REGISTRATION FOR SELECT AGENT PROGRAM
Investigators considering research involving Select Agents must contact the High Containment Lab Director (HCLD) prior to initiation. The HCLD can guide Investigators through the FSAP registration process and UCR requirements.
The following summarizes the Select Agent registration and compliance process at UCR:
- Notify HCLD/EH&S of your intent to possess, use or transfer Select Agents.
- Complete an update of the APHIS/CDC Form, Application for Registration: https://www.selectagents.gov/forms/index.htm
- Complete FBI Form FD961 and file with UCR EH&S for registration with applicable federal entity.
- Complete 2 sets of FBI fingerprint cards for initiation of background investigation check.
- Complete EH&S applicable training programs.
- Satisfactory completion of EH&S laboratory inspection of proposed work practices, safety equipment, and facilities, to evaluate compliance with CDC/APHIS, and Select Agent regulatory requirements (Safety and Security).
- Receive approval and authorization from EH&S, FBI, and the applicable governing body for you and each individual requesting access to Select Agents, for the proposed storage location, and research areas.
- Receive final approval and authorization from the IBC and the UCR High Containment Lab Oversight Group (HCLOG)
Your laboratory will be subject to EH&S and federal inspections or audits prior to initiation of work and at any time during your possession of Select Agents.
EXEMPT QUANTITITES OF SELECT AGENT TOXINS
Regulated toxins of biological origin (see table below) can be ordered, used, or maintained in the laboratory provided the total quantity per PI, for all areas under the PI’s control, does not exceed the limits posted in the table below. PIs are required to submit a BUA with UCR IBC for any quantity of biological toxins, including non-select-agent toxins and exempt quantities of select agent toxins.
The most current list of “Maximum Allowable Quantities” can be found here: https://www.selectagents.gov/sat/permissible.htm
|
HHS Toxins [§73.3(d)(3)] |
Amount |
|
Abrin |
1000 mg |
|
Botulinum neurotoxins |
1 mg |
|
Short, paralytic alpha conotoxins |
100 mg |
|
Diacetoxyscirpenol (DAS) |
10,000 mg |
|
Ricin |
1000 mg |
|
Saxitoxin |
500 mg |
|
Staphylococcal Enterotoxins (subtypes A, B, C, D and E) |
100 mg |
|
T-2 toxin |
10,000 mg |
|
Tetrodotoxin |
500 mg |
It is important to ensure that the total amount of toxin per PI in a laboratory is maintained below these limits at all times for exemption from Select Agent requirements. Failure to register a Select Agent is a criminal offense, punishable by up to five years in prison and/or $500,000 in fines.
POSSESSSION, USE, OR TRANSFER OF SELECT AGENTS
In order to possess, use, send or receive any amount of Select Agents or Select Agent Toxins in amounts equal to or greater than those listed, an institution and each individual who will have access to the Select Agent(s) should first satisfy each of the following requirements:
- Contact the UCR High Containment Lab Director to discuss scope of work and experimental plan (x2-4246)
- Register with U.S. governing bodies (CDC and/or APHIS) through UCR EH&S
- Official authorization granted for each individual requesting access to Select Agents provided by the U.S. Federal Bureau of Investigation, the applicable U.S. governing body, and UCR
- Complete all required trainings
Please note that violations of Select Agent rules and regulations can lead to severe criminal or civil penalties. Imprisonment and fines up to $250,000 may be levied against individuals who are found to be in violation of these laws.
DISCOVERY OF SELECT AGENTS
Notify EH&S immediately if:
- You identify any Select Agent pathogen or toxin listed on the current federal list that was not previously registered by your lab
- You discover a toxin not previously reported by your laboratory in excess of the federal maximum allowable quantities listed above
- You discover any unknown materials in your laboratory for assistance with identification
These discoveries should be reported to the applicable governmental institution(s).
INTRA-FACILITTY TRANSFER OF SELECT AGENTS
Select Agent pathogens and toxins may not be transferred outside of, to, or within UCR unless EH&S and federal approval has been granted. An intra-facility transfer is defined as the transfer of a Select Agent from EH&S and/or a federally registered Select Agent lab to a similarly registered laboratory. Select Agents may not be transferred to a laboratory that is not registered with EH&S and the applicable govern mental institution. Once approved, intra-facility transfers will be overseen by EH&S.
DESTRUCTION OF SELECT AGENTS
Select Agent pathogens or toxins may not be destroyed until EH&S and the applicable government institution have provided approval for the destruction. Once approval has been granted for the destruction of Select Agents, EH&S will officially assume possession of the material and record its destruction. The governing institution will alert UCR if witnesses are required.
If you have any questions regarding the UCR or Federal Select Agent process, please contact the EH&S Biosafety group at ehsbiosafety@ucr.edu.
XVI. Exposures and Injuries
EMERGENCY CONTACT INFORMATION
|
EH&S |
During business hours |
(951) 827 – 5528 |
|
UCPD |
Emergency |
911 |
|
UCPD |
Non-Emergency Non-Business Hours |
(951) 827 – 5222 |
REPORTING EXPOSURES AND INJURIES
Researchers exposed to biohazards in the lab should always seek immediate medical assistance. All biological exposures should be immediately reported to the person’s direct supervisor as well as EH&S. Exposures can be reported via the link on the main page for EH&S at https://ehs.ucr.edu.
Overt biological exposures with material containing biological toxins, recombinant organisms, human blood or tissue, or human infectious agents should be immediately reported to EH&S at (951-827-5528) or ehsbiosafety@ucr.edu. Additionally, hazards and incidents (including exposures and injuries) can be reported online at https://ehs.ucr.edu/.
High containment (BSL-3) lab researchers must immediately report any potential or overt biological exposure to the High Containment Lab Director at (tran.phan@ucr.edu or 951-827-4246).
Tips to Avoid Biological Exposures and Injuries
- Always pay attention.
- Do not work alone if possible.
- Do not work while fatigued. Take frequent breaks when the job task is repetitive.
- Read, understand, and follow all conditions described in the lab BUA, SOPs, and specific training.
- Always wear the appropriate PPE for the research including general lab attire (pants, closed-toe shoes)
- Share concerns or questions before you begin the procedure.
- Ensure equipment is being used in the manner for which it was designed and is functioning appropriately.
- When in doubt, contact your supervisor or EH&S before beginning work.
SKIN
Ask for assistance if necessary. Remove any contaminated clothing/PPE and dispose of as biohazardous waste. Wash the exposed area with soap and water for 15 minutes. For large volume exposures involving splashes to skin, remove as much contaminated clothing as possible and use the safety shower.
After washing, immediately report the incident to supervisor and EH&S. If needed, seek medical attention.
NEEDLESTICK, SHARPS INJURY, ANIMAL BITE/SCRATCH
Wash the wound with soap (or antiseptic) and water for at least 15 minutes. If the wound is bleeding, cover with sterile gauze after washing. Contact supervisor immediately. Depending on severity of wound or infection risk, seek medical treatment. Report the incident to supervisor and EH&S as soon as possible.
Sharps injuries involving recombinant organisms or human pathogens are required to be reported to the CDC and NIH. Sharps injuries need to be recorded and reviewed by EH&S. Researchers working with bloodborne pathogens or other potentially infectious material (OPIM) should refer to the UCR Exposure Control Plan for additional information.
AEROSOL
Notify others and leave the area immediately if infectious aerosols may still be present. Wait at least 30 minutes before returning. Post signage to prevent others from entering. Notify your supervisor immediately. Seek medical attention if necessary. Afterwards, report the incident as soon as possible to EH&S at https://ehs.ucr.edu.
MUCOUS MEMBRANE
If biohazardous material is ingested, do not induce vomiting. Flush area for 15 minutes with water. Eye exposures require use of an eyewash for 15 minutes. If possible, have another person assist you in finding and using the eyewash. Immediately seek medical assistance. Notify supervisor and EH&S as soon as possible.
XVII. Spill Procedures
In any spill scenario, the priority of actions is determined by the “PEP” rule – People, Environment, and Property. The highest priority is to provide aid to injured personnel and prevent spill area access to others. Next, action should be taken to prevent environmental damage if it can be done without endangering personnel. An example would be to prevent a potent toxin from entering a sanitary sewer drain by placing an absorbent in the flow path. Finally, action to prevent property damage should be taken if it can be done safely.
Small spills involving most biological materials used at UCR may be handled by trained laboratory personnel. If a spill is large or if laboratory personnel are uncomfortable handling the spill on their own, contact the following:
|
EH&S |
During business hours |
(951) 827 – 5528 |
|
UCPD |
Emergency |
911 |
|
UCPD |
Non-Emergency Non-Business Hours |
(951) 827 – 5222 |
Laboratory personnel should be prepared to clean up small spills of biological or biohazardous material. Keep basic clean up equipment on hand and ensure that all laboratory staff are trained to clean up spills. Researchers working with Risk Group 2 or 3 (RG2 or RG3) materials should prepare fresh 1:10 bleach solutions at least weekly for routine decontamination.
BIOLOGICAL SPILLS INSIDE BIOSAFETY CABINET
- Keep the biosafety cabinet on and do not lower sash.
- Don appropriate PPE for cleaning up the spill (gloves, lab coats, safety goggles, etc.).
- If there are any sharps, including broke glass, use forceps or tongs to pick up and place into biohazard sharps container.
- Place absorbent materials on and around the spill (e.g. paper towels). Always start placing down absorbent material at the edge of the spill and work inward.
- Apply an effective disinfectant such as freshly-made 10% (1:10 dilution) bleach solution to the spill and allow it to sit for the appropriate contact time, depending on the disinfectant. Add from perimeter and work toward the center. Avoid splashing or creation of aerosols.
- After appropriate contact time, carefully pick up absorbent materials and place into red biohazard bag.
- Disinfect the area again following steps 4-6. Note: If using bleach as a disinfectant, wipe the area with 70% alcohol or water afterwards to remove bleach residue to prevent corrosion of your biosafety cabinet.
- Wipe the area with paper towels to dry.
- Dispose all waste into a red biohazard bag.
- Remove gloves and put into red biohazard bag.
- Wash hands.
- Report the spill to your PI/Lab Manager/Supervisor.
BIOLOGICAL SPILLS OUTSIDE BIOSAFETY CABINET
- Notify all personnel in the area that a spill has occurred and evacuate everyone in the vicinity.
- Remove any contaminated clothing and wash exposed areas with mild soap and water for at least 5 minutes.
- Post signage and close door to room to prevent anyone from entering space.
- Wait 30 minutes to allow droplets to settle and aerosols to vent.
- Don appropriate PPE for cleaning up the spill (e.g. gloves, lab coat, safety goggles, etc.). Consider wearing shoe covers to prevent transferring/tracking of spill onto your shoes.
- if there are any sharps, including broken glass, use forceps or tongs to pick up and place into biohazard sharps container.
- Place absorbent materials (e.g. paper towels) on and around the spill. Always start placing down absorbent material at the edge of the spill and work inward.
- If it is a large spill, use larger absorbent pads (e.g. Pig Pads) or barriers to contain spill.
- Apply an effective disinfectant such as freshly made 10% bleach to the absorbent material covering the spill and allow it to sit for the appropriate contact time (e.g. 30 minutes for bleach). Add from perimeter and work toward the center. Avoid splashing and creation of aerosols.
- After appropriate contact time, pick up absorbent material and dispose into red biohazard bag.
- Disinfect the area again following steps 7-10.
- Consider using a mop with disinfectant to mop the floor around the spill area to disinfect any aerosols that might have landed on the floor around the spill.
- Remove gloves and place into red biohazard bags.
- Wash hands.
- Report the spill to your PI/Lab Manager/Supervisor and EH&S.
XVIII. Waste Management
Research with biohazardous materials generate medical waste which cannot be discarded in regular trash. Medical waste is defined by the California Medical Waste Management Act (MWMA) as
- Any biohazardous, pathology, pharmaceutical, or trace chemotherapy waste
- All sharps and any biohazardous waste from research involving the treatment, diagnosis or immunization of humans or animals
- Waste generated in autopsy or necropsy
- Waste generated in research using human or animal pathogens
- Laboratory waste such as human or animal specimen cultures that are infected with pathogens that are also infectious to humans
- Laboratory wastes from the production of bacteria, viruses, spores, discarded live and attenuated vaccines used in human health care or research
The CMWM requires anyone generating, treating, or storing medical waste to comply with the following procedures:
DISPOSAL OF SOLID BIOLOGICAL WASTE
1. Label a dual-test compliant (ASTM D1709 and ASTM D1922) red biohazard bag (orange biohazard bags are illegal in California) with PI name, building, and room number before filling it.
2. Place the waste in the red biohazard bag. Do not place glassware or anything that will puncture the plastic bag. Rigid objects such as transfer pipettes can be decontaminated by exposure to a 10% household bleach solution for at least 30 minutes.
3. Close the bag and loosely seal it with tape or twist ties.
a.) If autoclaving, place autoclave tape on the bag to confirm autoclave attainment of adequate sterilization conditions.
b.) If participating in the EH&S biohazardous waste pickup program, transfer the bag into another pre-labelled red biohazard bag (double bagged), close the outer bag and transfer to the provided biohazardous barrel.
4. Biohazardous waste bags should be stored in a labeled, rigid, leak-proof, puncture-proof container with a tight-fitting lid and biohazard symbol on all visible sides and top.
5. To dispose waste after autoclaving, take the biohazard bag directly to the EH&S provided biohazard waste bin.
6. All waste should be decontaminated and disposed within seven (7) days of generation if stored at a temperature above 0°C.
7. All waste should be disposed within 90 days if stored at or below 0°C.
LIQUID BIOLOGICAL WASTE
For liquid biological waste with no chemical hazards, liquid waste (cultures, stocks, and other regulated liquid waste) should be decontaminated by a 10% final concentration household bleach solution for 30 minutes minimum contact-time prior to disposal down the sink with copious amounts of running water.
BIOLOGICALLY CONTAMINATED SHARPS
Immediately dispose contaminated sharps into a sharps container that is rigid, puncture resistant, leak-proof on the side and bottom, portable, and labeled with the International Biohazard Symbol. EH&S provides biological sharps containers for all labs on campus. Contact EH&S at ehsbiosafety@ucr.edu to request additional sharps containers.
For proper removal of sharps container, create a waste tag by logging in to Waste Accumulation Storage Tracking Electronic (WASTe: https://ehs.ucop.edu/waste/#/), and create a “Biological” tag prior to generating any waste. Place the tag on the sharps container using hazardous waste envelopes (provided by EH&S). When the container is ¾ full, seal the container and update the sharps container tag status in WASTe to “Ready for Pick Up” and EH&S will pick up the container.
INFECTED OR TRANSGENIC PLANT MATERIAL
Infected or transgenic/recombinant plant material and soil should be disposed of in clear biohazard autoclavable bags. All infected/transgenic/recombinant plant waste should be devitalized (most commonly by autoclave) per any applicable permit conditions before disposal in EH&S provided biohazard waste bins.
NON-INFECTED AND NON-TRANSGENIC PLANT MATERIAL
Non-infected and non-transgenic/recombinant plant material and soil should be disposed of in clear autoclavable bags. All non-infected/non-transgenic/non-recombinant plant waste can be disposed of in regular trash.
ANIMAL CARCASSES
At UCR, disposal of animal carcasses is handled through the Office of Campus Veterinarian (OCV). Animal carcasses should be double bagged in red biohazardous bags, transported in leak-proof containers, and held in the freezer located in the vivarium until the next scheduled pick up from an approved vendor under contract with UCR. Follow procedures specified by the OCV.
XIX. Training
Safety training is available online via ucrlearning.ucr.edu. These cover general health and safety issues, and do not replace requirements for supervisors to train or make certain employees are trained in the lab-specific hazards of their workplace.
EH&S Required training:
- Biosafety Training (one time)
- Bloodborne Pathogen Training (if applicable) (annually)
- Aerosol Transmissible Diseases (if applicable) (annually)
- Hazardous Materials and Waste Management (annually)
- Fire Extinguisher Training (annually)
- Laboratory Safety Fundamentals (every 3 years)
Appendix A. Regulations and Guidelines
The following is a brief summary of regulatory authorities that regulate or established guidelines for the use of biological materials, infectious agents and recombinant DNA molecules.
The Biosafety in Microbiological and Biomedical Laboratories (BMBL), 6th edition, is the code of practice for biosafety – the discipline addressing the safe handling and containment of infectious microorganisms and hazardous biological materials.
Medical Waste Management Act 2017
The Medical Waste Management Act governs the proper handling, storage, transport, treatment, and disposal of all medical waste.
NIH Guidelines
The National Institutes of Health (NIH) developed the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines) to articulate safety practices and containment procedures for basic research involving recombinant or synthetic nucleic acid molecules, including the creation and use of organisms and viruses containing recombinant or synthetic acid molecules.
Federal Select Agent Program
The Federal Select Agent Program (FSAP) oversees the possession, use and transfer of biological select agents and toxins, which have the potential to pose a severe threat to public, animal or plant health or to animal or plant products. The Select Agent regulations fall under 7 CFR 331, 9 CFR 121, and 42 CFR 73.
Appendix B. Links and Documents
Plant Biosafety in Research Greenhouses: A Practical Guide to Containment