Nanomaterials Appendix C
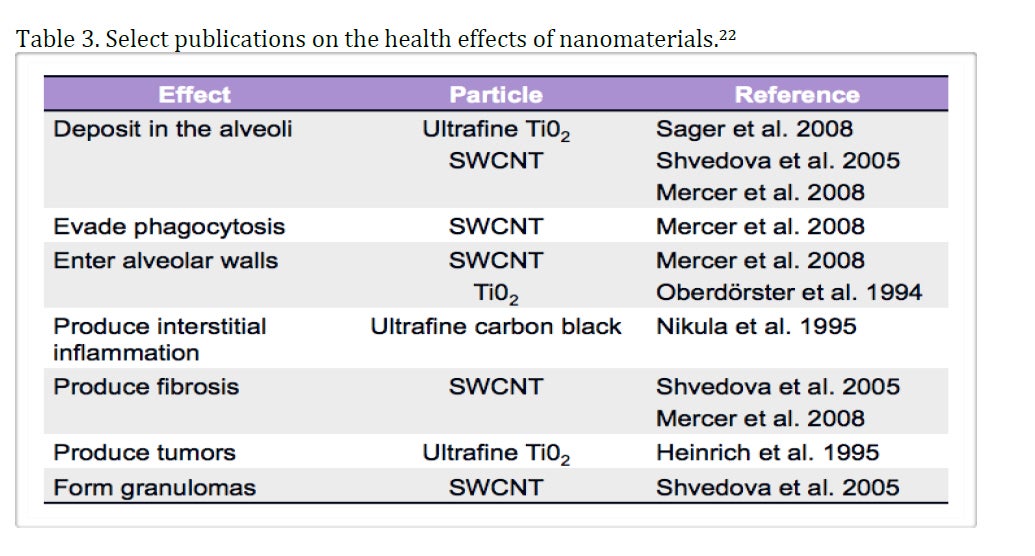
Health Effects
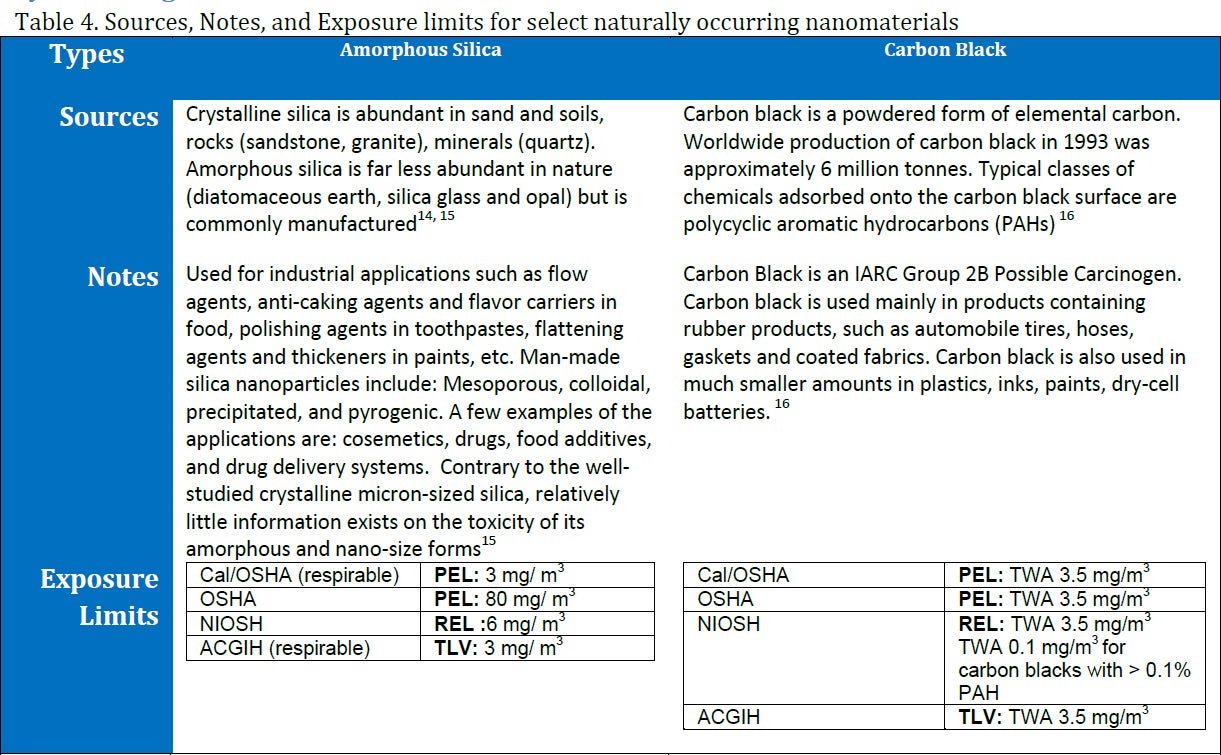
Naturally Occuring Nanomaterials
Regulations
Governmental Agencies and Relevant Legislation to Nanomaterials (as of 2011)
- European Union: Registration, Evaluation, Authorization, and Restriction of Chemical Substances, 2007 (REACH)
- US Environmental Protection Agency (EPA): Toxic Substances Control Act (1976)
- Cal EPA Department of Toxic Substance Control (DTSC): Assembly Bill 1879 and Senate Bill 509
- Occupational Safety and Health Administration (OSHA): Nanomaterials fall under OSHA General Industry Standards17
European Union | REACH18
REACH does not specifically refer to nanomaterials, but nanomaterials are included under the definition of a “substance” in this legislation. In general, REACH requires registration of substance manufactured or imported at 1 metric ton or more, but the initial (November 2010) registration deadline only applies to substances that are manufactured or imported in quantities at 1000 metric tons or more per year. This registration will provide information that is essential to understanding and evaluating the risks associated with specific substances, particularly nanomaterials, since value-chain information for most nanomaterials is currently lacking.
In addition, Classification, Labelling and Packaging of Nanomaterials in REACH and CLP, an amendment to REACH, specifies how hazardous substances must be handled within the EU; nanomaterials that fulfill the criteria for hazardous provided under this regulation must be classified, labeled, and packaged as such. Manufacturers and importers in the EU of nanomaterials that meet these criteria were required to notify the ECHA by January 2011.
An expert group that advises the European Commission on how to manage nanomaterials in accordance with REACH and CLP has released several documents, including:
Nanomaterials in REACH: Provides an overview of how REACH applies to nanomaterials
Classification, Labelling and Packaging of Nanomaterials in REACH and CLP
Describes how to classify nanomaterials in accordance with REACH and the CLP Regulation.
US EPA | Toxic Substances Control Act (1976)19
In the United States, Toxic Substances Control Act of 1976 (TSCA) is the primary federal legislation that regulates toxic substances. TSCA requires manufactures of new chemical substances to provide information to the EPA prior to manufacturing or introducing new substances into commerce. Under TSCA, the EPA has the authority to control substances that pose an “unreasonable risk to human health or the environment”. On April 4, 2012, the US EPA issued a Significant New Use Rule (SNUR) that states that “infused carbon nanostructures (generic) are subject to premanufacture notice (PMN).” This SNUR require persons who intend to manufacture, import, or process new carbon nanostructures to submit a Significant New Use Notice (SNUN) to EPA at least 90 days before commencing that activity. Additional rules related to other types of nanomaterials are expected to be issued from the EPA in the future and individuals wishing to use nanomaterials commercially are encouraged to check the EPA’s website for more current information: http://epa.gov/oppt/nano.
For an overview of how the TSCA impacts worker health related to nanomaterials see: Sayre, P., S. Prothero, and J. Alwood, Nanomaterial Risk Assessment and Management Experiences Related to Worker Health Under the Toxic Substances Control Act. Journal of Occupational and Environmental Medicine, 2011. 53(6 Supplement): p. S98-S102.
Cal EPA (DTSC) | Assembly Bill 1879 & Senate Bill 509
Two California Green Chemistry Initiative Statutes, Assembly Bill 1879 and Senate Bill 509 provide the Cal EPA/ DTSC with greater authority to regulate toxic substance in consumer products than the federal statues and to create an online Toxics Information Clearinghouse to provide Californians with information on hazardous chemicals. Although these statutes apply to chemical substance more broadly, DTSC has used this authority to create a chemical call-in program for some specific nanomaterials. Thus far, carbon nanotubes, nano cerium oxide, nano silver, nano titanium dioxide, nano zero valent iron, nano zinc oxide, and quantum dots have been included in the call in. The call in requires manufacturers and importers of these materials in the state of California to provide information on their products, including, but not limited to, known toxicological data and supply chain information.20, 21
Other Regulatory Drivers
OECD
Defines terms, and standardize protocols for safety testing. OECD Nanomaterials Website