Biosafety
What materials are under IBC purview?

Teaching and research laboratories that uses or stores biohazardous materials must submit a Biological Use Authorization application. The term “biohazardous materials” includes:
- Recombinant/synthetic nucleic acid molecules and genetically-modified organisms, as covered by the NIH Guidelines
- Potentially infectious organisms (typically Risk Group 2 or greater organisms) such as viruses, bacteria, fungi, or prions that can cause disease in humans or cause significant environmental or agricultural impact
- Select agents and select toxins, as covered by the CDC's Division of Select Agents and Toxins (DSAT) regulations
- Human and nonhuman primate materials (including established cell lines), as covered by the Cal/OSHA Bloodborne Pathogen Standard
- At its discretion or upon IACUC request, the IBC may also review protocols involving animals or animal specimens known to be reservoirs/vectors of zoonotic diseases
- Dual Use Research of Concern (DURC) and/or Gain-of-Function (GOF)
- Materials not within the IBC purview by regulation, that UCR may add to IBC oversight:
- Plant infectious agents or other infectious agents with potential environmental impact
- Exotic arthropods or microorganisms
- Biological materials requiring an APHIS, CDFA, EPA or other governmental permit
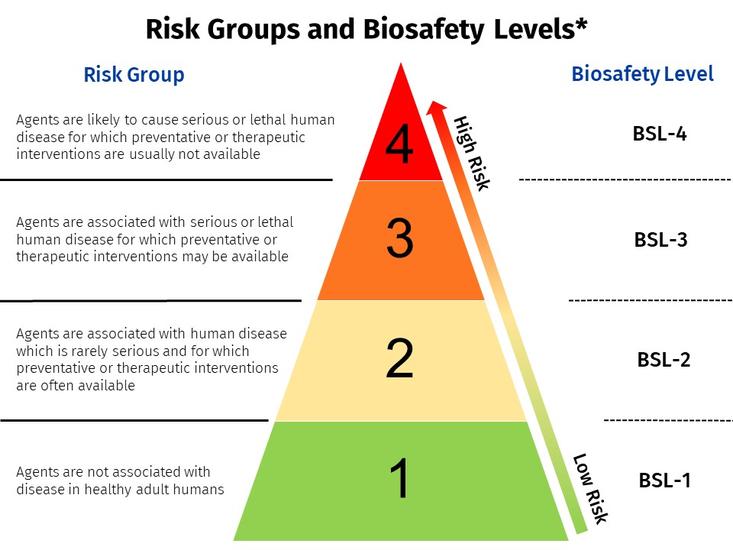
*Risk Group does not necessarily equate to the corresponding biosafety level. The IBC will determine the appropriate biosafety level based on risk assessment
Do I have to submit a BUA application?
A Biological Use Authorization (BUA) is required for every laboratory that uses or stores biohazardous materials. The term “biohazardous materials” includes:
- Recombinant/synthetic nucleic acid molecules and genetically-modified organisms, as covered by the NIH Guidelines
- Potentially infectious organisms (typically Risk Group 2 or greater organisms) such as viruses, bacteria, fungi, or prions that can cause disease in humans or cause significant environmental or agricultural impact
- Select agents and select toxins, as covered by the CDC's Division of Select Agents and Toxins (DSAT) regulations
- Human and nonhuman primate materials (including established cell lines), as covered by the Cal/OSHA Bloodborne Pathogen Standard
- At its discretion or upon IACUC request, the IBC may also review protocols involving animals or animal specimens known to be reservoirs/vectors of zoonotic diseases
- Dual Use Research of Concern (DURC)
- Materials not within the IBC purview by regulation, that UCR may add to IBC oversight:
- Plant infectious agents or other infectious agents with potential environmental impact, exotic arthropods, exotic microorganisms, BSL-1 microorganisms, biological material requiring an APHIS, CDFA, EPA or other governmental permit.
How do I submit a BUA application?
Log into https://app.riskandsafety.com
To submit an amendment or renewal to a previously approved BUA
- Your previously approved BUA(s) has been migrated over from the old BUA Portal. The BUAs are listed under your Workspace.
- Click on the BUA you wish to amend or renew.
- On the right hand column, select Amendment or Renewal.
- Review and edit each section as appropriate then click on Submit to Biosafety Review.
To submit a new BUA
- On the right hand side under Quick Links, select Begin a Biological Use Authorization.
- Answer and input information for all biological materials that you plan to work with. Fields with the red * are required and must be filled out.
- Depending on the materials you plan to work with, upload any required Standard Operating Procedures (SOPs) in the appropriate fields. Templates for the SOPs are linked within the BUA form.
- Fill in and edit each section as appropriate then click on Submit to Biosafety Review.
Tutorials
Tutorial story on how to submit new, amendment, and renewal BUAs for PIs. Includes the BUA approval process and how the IBC members and Chair access BUAs to review and approve them. Questions about the BUA process should be submitted to ehsbiosafety@ucr.edu.
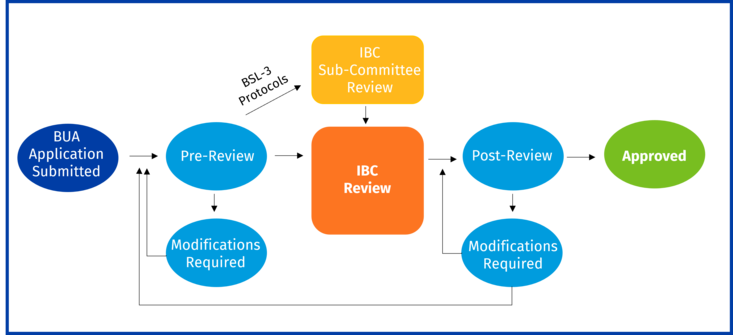
What is the BUA review and approval process?
All submitted BUAs are pre-reviewed by the IBC Administrator/EH&S (Biosafety Review) to ensure completeness of submitted information. The IBC Administrator will return the BUA to the PI if additional information is required to address any issues.
- If a BUA is determined to be exempt from IBC oversight, the IBC Administrator will notify the PI of this status. No additional action is required.
- Once the BUA passes Biosafety Review, it will go to full IBC Review or sub-committee review (BSL-3 protocols only) prior to going to full IBC review.
- Post review, the PI will be notified if modifications are required or if the BUA has received approval.
Additional compliance reviews that may be required:
Dual Use Research of Concern (DURC): DURC review life sciences research that can be reasonably anticipated to provide knowledge, information, products, or technologies that could be intentionally misused to pose a significant threat, with broad potential consequences, to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security. This applies to 15 agents and 7 sets of experimental effects of concern.
Institutional Animal Care and Use Committee (IACUC): The use of vertebrate animals (e.g., testing viable recombinant or synthetically modified microorganisms on whole animals) must be approved by the IACUC (via an AUP).
Institutional Review Board (IRB): IRB review of human protections may be required for some activities, such as the collection of blood or primary tissues.
Stem Cell Research Oversight (SCRO): SCRO review of the use or storage of human pluripotent stem cells is required.
Material Transfer Agreement (MTA): Some biohazardous materials (e.g., plasmids, vectors, viruses, human cell lines) cannot be obtained without a Material Transfer Agreement (MTA). The Research and Economic Development Office is required to review these contracts in accordance with UC mandated restrictions. It is recommended that you contact mta@ucr.edu as soon as you have determined you will be obtaining materials from an external source.
Required Training
BUA approval is contingent upon completion of required trainings. All personnel that may be present in the laboratory, whether they directly work on the project or not, must complete the required trainings. This is to ensure that any personnel who may potentially be exposed to the proposed work materials have received adequate training. The required trainings are:
- Biosafety (must be completed once)
- Laboratory Safety Fundamentals (every 3 years). Note: Safety Orientation does not fulfill the requirement for Laboratory Safety Fundamentals
If the proposed project works with the following materials, additional trainings required are:
- Bloodborne Pathogens Online (annually) (for projects working with with human/non-human primate materials or other potentially infectious materials)
- Aerosol Transmissible Diseases (annually) (for projects working with aerosol transmissible diseases or pathogens – laboratory as listed in the Aerosol Transmissible Diseases Standard
All trainings can be found in the UC Learning Center: https://ucrlearning.ucr.edu/.
Schedule of IBC Meetings & Submission Deadlines for Full Committee Review
|
For BUA applications that require full committee review (BSL-2 and BSL-3) Submit via RSS by 3:00pm Submission Deadlines |
IBC Meeting Dates |
| May 1, 2025 | May 22, 2025 |
| June 5, 2025 | June 26, 2025 |
| July meeting tentatively canceled | July meeting tentatively canceled |
| August 7, 2025 | August 28, 2025 |
| September 4, 2025 | September 25, 2025 |
| October 2, 2025 | October 23, 2025 |
| November 13, 2025 | TBD -- Combined Nov/Dec due to holidays |
| January 1, 2026 | January 22, 2026 |
| February 5, 2026 | February 26, 2026 |
| March 5, 2026 | March 26, 2026 |
| April 2, 2026 | April 23, 2026 |
| May 7, 2026 | May 28, 2026 |
| June 4, 2026 | June 25, 2026 |
Timelines
The IBC normally meets on the fourth Thursday of each month. For a BUA application to be placed on the agenda for a meeting, the PI should submit the BUA at least three weeks before the scheduled meeting and adequately address any issues raised during the pre-review by Environmental Health & Safety (EH&S). If any major issues are unresolved or key documents are still pending, the BUA review may be postponed for a future meeting. PIs are encouraged to submit renewal applications at least two meetings before the BUA expiration date to allow sufficient time for pre-review and to address any issues.
Non BSL-3 BUAs are approved for a three-year period while BSL-3 BUAs are approved for a one-year period. RSS Biosafety will send email reminders to the investigators to submit renewals. However, it is the responsibility of the investigator to ensure that an active approved BUA is maintained. This responsibility includes the submission of amendments when changes to the research are required.
IBC Meeting Minutes
As required by NOT-OD-25-082, effective June 1, 2025, approved IBC meeting minutes may be accessed in the IBC Meetings SharePoint Folder.
Manuals
- Biosafety Manual
- Bloodborne Pathogen and Aerosol Transmissible Disease Exposure Control Plan
- Medical Waste Management Plan
Forms
- Autoclave Use Logs
- Autoclave Monitoring Monthly Testing Log
- Biological Use Authorization (BUA) Application
- Biohazardous Materials Signage (BSL-1/ABSL-1/ACL-1/BSL-1P)
- Biohazardous Materials Signage (BSL-2/ABSL-2/ACL-2/BSL-2P)
Guides
- Biosafety Cabinets
- Biohazardous Waste Pick Up Program
- Biohazardous Medical Waste Disposal Requirements
- Disposal of Sharps Spotlight on Safety
- E. coli Strains & NIH Guidelines Guidance
- Guidance for PIs for BSL-3 Research Projects
- Hepatitis B Vaccine Guidance
- Isoflurane Anesthetic Gas Safety Guidelines
- Microtome Safety Program
- Needle and Syringe Safety
- Select Agent Toxins Exempt Quantities
- Spill Clean Up
- SARS-CoV2 Guidelines
Policies
Use the following SOPs as templates, and personalize for your laboratories needs. The SOPs may also be referenced in BUA and AUP submissions.
Biosafety
- PI-Specific SOP Template
- Biological Materials Packing and Transport SOP
- Biological Spill Cleanup SOP
Biological Use
Animal Use Protocol (AUP) Section 12 - Hazardous Materials Information
- Adeno-associated Virus (AAV) with Helper Virus or Containing Oncogenes/Toxins
- Adeno-associated Virus (AAV) without Hazardous Cargo Genes (oncogenes, toxins)
- BrdU and EdU
- Clozapine N-oxide (CNO)
- Fatal Plus (Sodium Pentobarbital solution)
- Formaldehyde/Formalin/Paraformaldehyde
- Isoflurane
- Ketamine/Xylazine
- Streptozotocin (STZ)
- Tamoxifen
- Tricaine Methane Sulfonate (TMS)/MS-222
- Appendix A template
The import, interstate and intrastate movement, and export of infectious agents and/or biological materials suspected to contain hazardous agents or have an environmental impact are tightly regulated by Federal and State agencies and may require permits. These permits minimize the risk of inadvertent release and the inappropriate use of these materials. Compliance with multiple regulatory agencies may be required in some cases, depending on the material.
It is important to remember that most permits are issued to the individual, and not to the institution. Therefore, the Principal Investigator (PI) is responsible for determining permit requirements from the relevant agencies, applying for the permit, and ensuring that all permit conditions are followed. If a permit is required, the recipient of the regulated material must obtain the permit prior to possession or transport. If shipping to another individual or entity, a copy of the permit must be obtained from the recipient and included in the shipping package.
The permit application process may take several weeks to months. Some permit applications will also require an inspection of the laboratory facilities. PIs should plan to allow for sufficient processing time before the materials are needed.
Failure to comply with regulations when transporting regulated biological materials may result in shipment delays or confiscation of samples at the port of entry by U.S. Customs or the U.S. Public Health Service Division of Quarantine, refusal of the shipment by carriers, and be subject to fines and/or criminal penalties by the federal agencies or law enforcement.
How EH&S can help with the permitting process
Contact EH&S Biosafety prior to your application submission. For PIs with existing permits, forward a copy of the permit to us. EH&S can provide assistance in the following ways:
- Help in determining if a permit(s) is required for the material you would like to import/move/export.
- Provide assistance with application form submission to ensure that all pertinent information is included for an efficient review of the application to prevent delays in approval.
- Help develop and implement any biocontainment or biosecurity requirements necessary for permit approval and maintenance.
- Assist in preparing, attending, and responding to laboratory inspections by permitting agencies.
- Work with the various agencies throughout the permitting process and assist with any communication regarding permit conditions.
- Help ensure that permit conditions are met to maintain compliance and approval.
Click here for more information on individual permit types.
Materials Requiring Export Control
Federal laws govern the conditions under which certain information, technology, technical data, materials, commodities, and equipment can be exported to a foreign person, entity, or country, or to a foreign national within the United States. The primary goal of export control is to protect US national security and promote US foreign policy objectives. For more information on export control requirements, visit the UCR Export Control website at https://exportcontrol.ucr.edu/ or email exportcontrol.ucr.edu.
UCR Resources
- SARS-CoV-2 Research Materials Policies & Guidelines
- SARS-CoV-2 Research Laboratory Biosafety Guidelines
- Guidance for PIs for BSL-3 SARS-CoV-2 Research Projects
- BUA Application Tutorial
- IBC Charter – Committee Roles and Responsibilities
- IBC Reporting and Duties
- LMS Training Instructions - IBC Members
- LMS Training Instructions - Investigators
- Research Approval and Training Requirements
- Environmental Health & Safety - Training
- ORI policies
- ORI guidance
Government Resources
- NIH - Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines)
- CDC/NIH - Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th
- California Occupational Safety and Health Administration - Bloodborne Pathogen Standard California Code of Regulation, Title 8 §5193
- Centers for Disease Control and Prevention (CDC) - Select Agent Program
- American Biological Safety Association (ABSA) - Risk Group Database
- The World Health Organization (WHO)
- Public Health Agency of Canada
- NIH Grants Policy Statement
- NIH Office of Science Policy on NIH Guidelines
Additional Resources
- Biosafety in Microbiological and Biomedical Laboratories (BMBL), 6th ed.
- ABSA Risk Group Classifications
- Plant Containment Guide
- Public Health Agency of Canada - Pathogen Safety Data Sheet
- Nuaire - Biosafety Cabinet
Related Links
- Institutional Registration and Accreditation Numbers
- Research Integrity
- Research Misconduct (RM) Allegations
- Responsible Conduct of Research (RCR) Requirements
- Research Participant/Subject Resources
- HIPAA & Human Subjects Research
- IRB Workshops & Outreach